on behalf of the
Molecular and Cellular Research Team
Agriculture and Agri-Food Canada, Guelph, Ontario
|
http://distans.livstek.lth.se:2080/foodbugs.htm because occasional failures of that server made it inaccessible. The original URL will function as soon as that server is repaired. The host apologizes for any inconvenience. Thank you. Food-borne pathogens are still the world's major cause of human health problems. Even in a technologically developed country such as Canada, there are 2.2 million cases of food-borne illness per year costing an estimated $1-2 billion in lost work and medical expense. In the USA, there are over 80 million cases and 9,000 related deaths each year.
For fifty years, antibiotics have been used to treat food-borne bacterial infections. They are also added to animal feed as growth promoters. The exposure of bacteria to antibiotics over the years has resulted in the selection of resistant types. Illness will not be so easily controlled by antibiotics in future, as resistance genes spread geographically and between bacterial species without human intervention. This comes at a time when ideas and biochemical possibilities for replacement antibiotics seem to be in short supply. In Canada, there is an additional concern. The number of older and immuno-compromised people in the population, who are most susceptible to food-borne illness, is increasing. Thus, we have the daunting situation that new and virulent pathogens continue to arise against which neither the immune system nor antibiotics are effective. The food industry is aware of its responsibility. Producers and processors are uniting to adopt the most effective methods to monitor pathogens 'from farm to fork' under the HACCP (Hazard Analysis at Critical Control Points) program. Routine spot-checks will be carried out to detect and enumerate specific pathogens at all stages of food production, processing and distribution to maintain standards of hygiene. In the USA, the benefits of HACCP for meat and poultry, in terms of medical cost savings and reduced time off work, is estimated to be more than $170 billion over the next 20 years. This would amply offset the cost of implementing HACCP estimated at $1.1-1.3 billion over the same period. While the implementation of HACCP is very important, alone it is not enough. In order to protect Canadian food, the industry, and consumers, the development of non-antibiotic therapies to hold the line against emerging pathogens is urgently needed. The suppression of pathogens in animal reservoirs will be critical. It is not an unreasonable long-term objective to control human pathogens, like Salmonella, Campylobacter, and EHEC, in farm livestock.
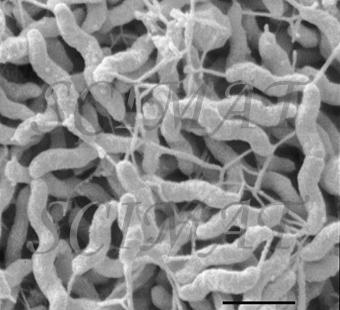
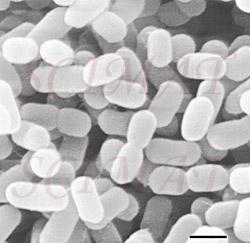
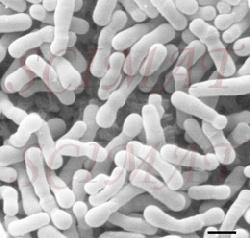
However, there is a growing evidence that treatment of livestock with selected bacteria can reduce the number of cells of Salmonella, Campylobacter and, possibly, E. coli O157:H7 both in the intestines and in the excrements. Already probiotic preparations are on the US market as prophylactic inoculants to treat chicks against Salmonella. For example, MS BioScience's 'PREEMPT' (Madison, Wisconsin) is a probiotic spray mixture of 29 bacterial species which are effective collectively. The microbial community of the healthy gut is dense, diverse and highly active; over 400 species and a trillion bacteria per gram-contents is not unreal. Traditional diagnostic assays and growth tests using selective culture media have sketched a crude picture of population structures. But the real diversity and complexity has been greatly underestimated, for many bacteria of the gut do not grow in isolation in the lab and this fact was not appreciated at the time. Recent developments of molecular techniques (for example, DNA probing, sequencing, marking genes, access to databases, and PCR) have made it possible to study the diversity, succession and gene activity of the gut microbial community as never before, including its non-cultivable members. It is now possible to explore the true complexity and underlying interactions at the cellular and molecular level. This knowledge is essential for the logical development and patenting of novel probiotic bacteria. Under natural conditions, the gut microbial community of a free-range chick develops rapidly from the time of hatching. The chick is soon infected with bacteria from its mother, fellow chickens and the environment. Contrast the chick reared under commercial conditions, there being no contact with older birds. Hatched under near-sterile conditions, fed antibiotics, brooded in clean barns disinfected to control pathogens, where is the chance for a young chick to meet eligible bacteria and develop a healthy meaningful relationship? Deprivation leaves chicks more susceptible to colonization by Salmonella than perhaps luckier chicks, which are naturally or artificially inoculated with probiotic bacteria. Normal microfloral development proceeds with a succession of different bacterial species becoming dominant in the gut until a stable, climax situation is reached. The first waves of colonizers are facultative anaerobes, coliforms and streptococci and some clostridia. These are displaced by lactobacilli, first in the crop and then the small intestine. As conditions become favourable in the chick's caeca, obligate anaerobes move in and take over. It may take 4-6 weeks for a stable climax community to be established. How best one might insinuate probiotic bacteria into this system is one kind of research problem; knowing which are the best candidate probiotic bacteria to use is quite another. Another way pathogens might be controlled is by phage therapy. Bacteriophages (bacterial viruses or phages) were discovered 50 years ago and immediately their potential as specific non-toxic antibacterial agents was recognized. (To learn about them, click Index, then B, and finally Bacteriophage at this site.) However, with the advent of antibiotics, research into phage therapy was swept aside. Today, in anticipation of the exodus of antibiotics, research has begun anew in several laboratories.
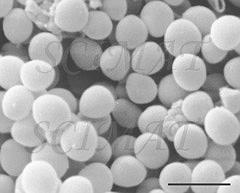
Infected cows have conventionally been treated with antibiotics, which are administered into the udder, or systematically. But, as is the case with other bacterial species, new forms of S. aureus have emerged that resist conventional antibiotics. Antibiotic treatment was already being questioned because of a deterioration in the general health of treated cows. Moreover, milk was being contaminated with antibiotic residues making it unacceptable to consumers. Without effective treatment, cows with S. aureus are a potential source of infection for other cows, and the incidence and economic impact of the disease can be expected to increase.
|