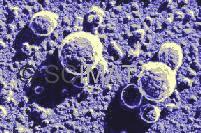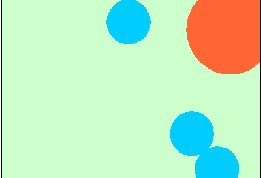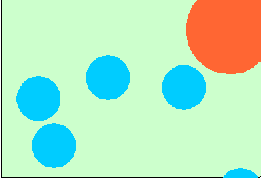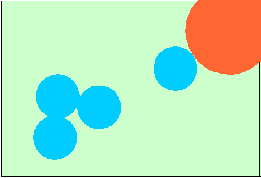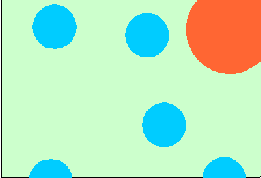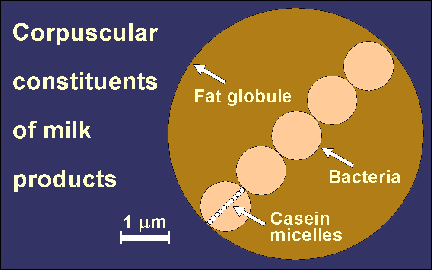
Everybody knows that milk is liquid. But why is it opaque and white? Or does it have a yellowish tint? Why and when does it curdle? What is actually curdling? If yogurt and cheese are both made from milk, why are they so different from each other? Is it true that there are bacteria in both yogurt and cheese? Why are they there? Answers to these questions may already be found on the Internet. Milk is produced by all mammal females to feed their newborns and the liquid form is most convenient. This means that the concentration of nutrients is relatively low compared to foods consumed by adults. Cow milk contains 3 to 4% protein, 4 to 4.5% fat, and approximately 4.5% milk sugar (lactose). It is opaque because it contains very small particles of casein (milk protein), approximately 100 nm in diameter. (That is 1/10 of a micrometer; 1 micrometer is 1/1000 of a millimeter). It also contains larger fat globules. These particles do not allow the light to pass through (except for a very thin layer) and scatter it in all directions making the milk to look opaque. The fact that all wavelengths of visible light are scattered at the same extent makes the milk white. Whereas fat globules may be observed under a light (optical) microscope, casein particles are too small to be seen using this kind of microscope. They are also too small to be captured on most laboratory filters but they may be concentrated using various ultrafiltration techniques. This is now done on a commercial scale. The yellowish tint of milk comes from fat. It is in the form of much larger globules, with an average of 3 to 5 micrometers in diameter as shown by the diagram at the title. Some large fat globules may be up to 15 micrometers in diameter. Cows which are fed a carotene-rich feed, such as grass or hay, pass the lipophilic, orange-coloured pro-vitamin A into the milkfat and that makes the milk slightly yellowish. Fresh milk contains lactic acid bacteria and other microorganisms as contaminants. Their dimensions are also shown in the diagram. Pasteurization (heating; usually at 63°C for a minimum of 30 minutes) kills the bacteria and makes the milk safe and more stable. This means that the shelf life of the milk is increased. A considerably more severe heating (e.g., 110°C for 30-40 min., 130°C for 30 seconds, or 150°C for <1 s) leads to the production of UHT or sterilized milk with a markedly extended shelf life. Milk coagulates (curdles) particularly when it is acidified - either by lactic acid produced by lactic acid bacteria or by stirring an acid (such as citric acid) into it. Milk also contains ingredients so small that they cannot be seen - not even under an electron microscope - yet they are very important both in the diet and in the manufacture of milk products. They are whey proteins (α-lactalbumin and β-lactoglobulin), the milk sugar called lactose, and minerals and vitamins.
Casein micelles consist of smaller particles called submicelles. A model explains the ultrastructure of the micelles and their aggregation. Casein micelles form the base of the majority of milk products such as yogurt and cheeses. Two major whey proteins are α-lactalbumin and β-lactoglobulin. Both have a high nutritional value partly because they contain sulfur-containing amino acids, cysteine, cystine, and methionine. Alpha-Lactalbumin An interesting structural property of α-lactalbumin has recently been discovered in Sweden by Dr. Catharina Svanborg. Her research group studies bactericidal properties of human milk. To test the way, in which breast milk prevents pathogenic bacteria from infecting other cells, Dr. Svanborg has also used cancer cells. In her experimental setting, the nuclei of the cancer cells started to shrink and die under the effect of breast milk. Their "programmed" death in this case is called apoptosis - the cells fall apart to be recycled by the body. The substance which causes the death of the cancer cells has been identified as α-lactalbumin. This protein may be converted into a protein which induces apoptosis. Proteins are long chains consisting of individual 'links' called 'amino acids'. There are 20 different amino acids which may form different sequences. The human body contains about 50,000 different proteins. In their native state they are very flexible. When α-lactalbumin is completely folded, it serves as a nourishment. When it is partially unfolded, it destroys pathogenic microorganisms and cancer cells. In the case of α-lactalbumin there is an interesting connection between its nutritious and body-protective roles. Apart from emphasizing the importance of nursing, this study shows a new way of fighting infections and malignancy.
Beta-Lactoglobulin is the most abundant protein in whey (about 6 g/l) and is responsible for functional properties such as the ability of whey proteins to form a gel on heating. Fat globules in fresh milk are encased ('packaged') in membranes which are lipophilic ('fat loving') on the inside where they are in contact with fat and hydrophilic ('water loving') on the outside where they are in contact with the aqueous medium. Membranes prevent the fat globules from coalescing (aggregating). Since fat has a lower specific gravity (is 'lighter') than the milk serum, fat globules slowly raise to the milk surface. Thus, even under primitive conditions, fat can be collected (skimmed) from the milk. The fat thus collected is called 'cream' and the milk deprived of fat is called 'skimmed milk' or 'skimmilk'. Fat can be prevented from raising in milk by breaking the fat globules into much smaller droplets so that they would be subjected to the Brownian motion. [In another model, the blue large particle may be seen as representing a fat globule and the minute red balls may be considered as casein micelles.] The process which makes this possible is called homogenization of milk. It consists of breaking the original large fat globules into much smaller droplets, 0.1 to 2 micrometers in diameter. Homogenization has two effects:
The disintegration of the large fat globules results in a 6-fold increase in the total surface of all fat globules in a unit volume. The 'naked' fat droplets immediately attract various milk proteins which then form artificial membranes and, thus, even the fragmented fat globules are protected from aggregation.
Comminuted meat is held together in meat products such as wieners and salami with the help of so-called meat binders. They consist of cereal products (wheat flour, baked crumb, and starches), milk products (nonfat dry milk), salt, and spices (which may include mustard flour). ["Kosher" meat products must not contain any milk solids.] Meat binders must not introduce any undesirable properties into the finished meat products. Milk powder is one of the most important ingredients. However, this powder should not contain buttermilk solids because they could alter the flavour of the meat products. Companies which produce meat binders are, therefore, careful about the quality of the milk powders which they use and want to be sure that there is no buttermilk present in them. Chemical analysis cannot be used to detect small amounts of buttermilk solids in the bulk of skimmilk powder because the major ingredients in both products are the same. During drying, buttermilk solids may get into skimmilk solids unintentionally, if sweet uncultured buttermilk is added to whole milk destined for cream separation in order to retrieve the residual fat from the buttermilk.
An electron microscopy procedure has been developed for the detection of buttermilk solids. The analytical principle is based on the peculiar composition of buttermilk. It is a byproduct of butter. Butter is made from cream, i.e., from milkfat globules. Each globule is covered with a membrane and the membranes on all fat globules break as the cream is churned to produce butter. A small proportion of the membrane fragments goes into the butter but a considerably larger part remains in the aqueous phase and forms "churn" buttermilk. Buttermilk has a high nutritional quality and is used in a variety of food products, particularly baked goods, where it improves their properties. It is not desirable in meat binders. Transmission electron microscopy (TEM) can be used to detect milkfat membrane fragments in milk solids. They may even be detected in the finished meat binders. Low-speed centrifugation (415 g for 30 min) of an aqueous suspension of the binders separates coarse particles such as spices, flour, and starch. Only milk solids remain in the supernatant. Ultracentrifugation at high speed (8x104 g) causes even the casein micelles and the milkfat globule membrane fragments to sediment. The pellet is fixed, embedded in a resin, and the resulting bloc is sectioned, stained, and examined. Pure skimmilk powder reveals only the presence of casein micelles. Casein micelles are also present in buttermilk solids and there are fat globule membrane fragments clearly visible in addition. Studies in greater detait revealed that as little as 1 part of buttermilk solids can be detected in 20 parts of nonfat milk solids using TEM. |
| Updated: May 16, 2012. Autor: M. Kalab |
| Home |