| Microscopy | Foodmiblog: M. Kaláb | Surface replication |
| Yogurt | Foods & bacteria | Bacteria in foods |
| Milk | Sticky tape | Anaglyphs: 3D SEM |
| Guests 3 | Food Struct. Journal | Bacterial filters |
Home
| Updated: August 25, 2011.
New information |
|
Cheeses are the most common dairy products. The basic procedure is simple and is based on spontaneous processes which, thousands of years ago, probably led to the development of cheese. Milk from various animals, particularly cattle, buffaloes, goats, and sheep is used to make cheese. One of the best descriptions of cheesemaking procedures is easy to follow. Various books may be found in libraries. Some scientific journal papers dealing with the development of structure in cheese are richly illustrated with micrographs, e.g., in
To make cheese, milk is curdled using a bacterial starter culture and an agent called rennet present in the tissue of the calf stomach. Rennet (chymosin) is a proteolytic enzyme and its role in cheese making is to destabilize casein micelles and make them to coagulate. Similar enzymes are also found in digestive tract tissues of other animals including chickens. Global shortage of animal-based enzymes and various kinds of aversion of some people to such sources, have led to the use of proteases isolated from plants and microorganisms to make cheeses, some of which are called vegetarian cheeses.
The proteases break down κ-casein present on the surfaces of casein micelles in milk. Deprived of its protective action, casein micelles coagulate and form a gel. When examined by electron microscopy, the coagulum consists of casein micelle clusters and short chains. They encapsulate fat globules - the natural large corpuscular particles present in milk. Void spaces in the casein matrix are filled with the liquid milk serum called whey which is a solution of lactose, minerals, and vitamins, and a suspension of whey proteins. The subsequent steps in the cheese manufacture are aimed at separating the curd from the whey and ripening the curd into cheese. There are many photographs showing, how cheese is being made.
Freshly coagulated milk is cut or broken into smaller particles using wire knives, stirrers or other tools and the cut or broken milk gel is slowly heated and stirred. Casein micelle clusters gradually shrink as they expel the whey and the protein matrix becomes compacted.
Another milk component - fat globules - stays with the proteins in the curd. A small number of fat globules, which were exposed during curd cutting, are washed away with the whey. The micrograph at left shows an early stage of milk coagulation. Fixation in a glutaraldehyde solution preserved the solid constituents allowing water to be removed to show the microstructure of the milk coagulum.
Homogenization of milk is a process during which large fat globules are disintegrated into considerably smaller particles. Their total surface is up to 6-fold larger than was the total surface of the original fat globules. Since the original fat globule membranes were fragmented by homogenization, there is a large area of unprotected exposed fat surface. Consequently, the small fat particles react immediately with any available proteins in the medium until all bare fat is again well covered. Even entire casein micelles are used for this purpose. Homogenized milk produces a different kind of curd. It is firmer than the curd made from nonhomogenized milk provided that all other parameters have been left unchanged (enzyme, total solids, pH). This phenomenon was known even before the use of electron microscopy, but now the reason for the difference is clear: The minute fat globules with casein micelles anchored on their surfaces have become part of the protein matrix. Fat is no more an inert inclusion but has become a structural constituent. This example is one of many which show the benefits of electron microscopy in elucidating interactions among food constituents and the development of food microstructure.
Each individual parameter used during the cheese manufacture has some effect on the cheese produced. This is probably one of the reasons for the great variety of cheeses. On the other hand, it is a great challenge for the cheese producer to keep sensory attributes of a particular product constant in a world where, for example, the original rennet has to be replaced with a similar product from a different source because there is a global shortage of calf stomachs or because the consumers request that no constituents of animal tissues be used in cheese production. Enzymes with a high proteolytic activity may be efficient in quickly curdling the milk but they may also disintegrate a large proportion of the milk proteins which would consequently be lost for the cheese production. High proteolytic activity may also lead to a weakened casein matrix and alter the characteristic consistency of the cheese. Similar constrains are encountered quite frequently. However, new technologies and new ingredients offer new directions in cheese manufacture. Examples will be presented as this home page is further developed.
Rennet is added to milk along with a lactic bacteria culture. The role of the bacteria is to assist curdling by decreasing pH of the milk. This is achieved as the bacteria oxidize lactose into lactic acid. After the whey had been removed and the curd salted and pressed, the next stage - ripening - takes place at a lower temperature for several weeks or months. This is the time when bacteria slowly degrade the milk proteins and produce substances which give the cheese its characteristic structure (carbon dioxide eyes in Swiss-type cheeses) and flavour (e.g., a low concentration of propionic acid), The great variety of cheeses is made possible by the combinations of many varieties of specific bacteria. However, some cheeses are made with Penicillium moulds (fungi) such as Penicillium camemberti and P. roqueforti. rather than bacteria. A small group of cheeses (Paneer, Queso Blanco, White cheese) is made by coagulating milk while it is hot, with an acid, such as lactic acid. Such cheeses are not ripened.
Some cheeses (Indian Paneer cheese, South American Queso Blanco cheese, American White cheese, North American Ricotta cheese) are made by coagulating hot milk with an acid and separating the curd from the whey.
How the microstructure develops has not yet been fully explained but it is known that three essential conditions must be met: As the name indicates, Cream cheese is made from pure cream or from mixtures of cream and milk. It has a rich, mildly acidic flavour and a smooth buttery consistency. In the traditional system of manufacturing, the cream mixture is pasteurized, homogenized, and coagulated using a lactic bacterial culture. The curd is then heated to 52-63°C, drained, and hot-packed or cold-packed. This kind of manufacturing procedure yields whey which has to be disposed off. In a newly formulated method of 'whey-less' manufacturing, the cream-and-milk mixture has the total solids composition of the cheese. The mixture is also pasteurized, homogenized, and incubated with a lactic bacterial culture at ~30°C. Then the solidified mixture is homogenized again and packed without cooling. Products which have not been made by the traditional procedure may not be called 'cream cheese' and terms such as 'cream cheese food' or 'cream cheese spread' are used.
Another new procedure has been suggested by H. W. Modler. Curd is first produced by coagulating hot milk with a 2.5% citric acid acid solution until pH of 5.3-5.5 is reached. The whey is drained off. Whey proteins are retained in the curd depending on how high the milk was heated prior to coagulation. The curd is mixed with high-fat (58%) cultured cream and the mixture is homogenized at ~70°C and the resulting cream cheese spread is hot-packed. Structural differences between the cheeses are best observed using TEM of thin sections. This technique makes it possible to examine the interior of the cheese particles whereas SEM shows surfaces. This is useful, too, because surfaces may be formed by breaking (fracturing) cheese particles; the structure of the protein matrix may thus also be observed. Preparation of the cheese specimens for electron microscopy may preserve the fat globules or remove them. In the traditional Cream cheese, TEM reveals a very high fat content in the form of minute fat globules. Their surfaces are covered with protein particles. The protein frequently covers fat globule clusters rather than each individual fat globule. The newly formulated Cream cheese spread structure is different. This is noticeable at the first glance: the fat is present in the form of relatively large fat particles which are not associated with protein. Protein is relatively evenly distributed through the body of the spread in the form of small clusters attached to the fat particles at random. Cream cheese spread made by homogenizing high-fat cream with fresh curd differs from the products mentioned above and reflects the manufacturing procedure. Fat is mostly in the form of small globule clusters and the protein is in the form of relatively large particles. The structure of the curd also reveals its origin - acid-induced coagulation of hot milk to pH 5.5. The 'core-and-shell' ultrastructure of the casein particles is preserved (figure above at right), although the curd undergoes homogenization during production.
Information on Cream cheese products presented in this section is based on earlier experimental work described in several papers co-authored by D. A. Froehlich, M. Kaláb, H. W. Modler, and A. G. Sargant, in Food Structure and in Milchwissenschaft 40(4):193-196 (1985) (Milk gel structure. XV. Electron microscopy of whey protein-based Cream cheese spread).
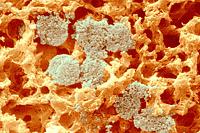
Electron microscopy has also been used in studies of low-fat or fat-free cheeses, where fat has been replaced with one of the so-called fat replacers. They may be based on proteins, polysaccharides, or even on indigestible fats and oils. Their action is based on the fact that our tongue receives stronger signals about the dimensions of the particles than about their chemical nature. Particles 1 to 3 µm in diameter are perceived as fat. In the micrograph at left, a protein-based fat replacer has been incorporated in cheese. It affects, because of its protein nature, only sensory attributes and instrumental measurements of the cheese. Protein- and polysaccharide-based fat replacers do not melt and their use is limited to specific situations, for example frozen desserts, salad dressings, and some other applications. In the low-fat cheese featured at left, a fat substitute based on protein (light green-coloured globular aggregates of microparticulated protein) was used to replace a small portion of milkfat. Fat globules originally present in the cheese were extracted from the sample while it was prepared for scanning electron microscopy. Initially they occupied the spaces which now appear empty in the protein body of the cheese. The low-fat cheese sample shown at left was freeze-fractured. This procedure makes it possible to fracture (break) even minute particles and study their internal structure by scanning electron microscopy. Additional scientific papers are being processed for this page. Images of fat substitutes used in cheese, the structure of cheeses made without the aid of microorganisms, Cottage cheese and other cheeses such as Mozzarella will be gradually presented at this site. Processed cheese is another important dairy product in which interesting discoveries have been made using electron microscopy.
Brick cheese vs. Cheddar cheese
Differences between cheeses can not only be tasted but also seen, because manufacturing processes impart special features on the cheese microstructure. To make cheese, curdled milk is cut using steel wire knives or the milk gel is broken into small particles using a propeller stirrer. Subsequent heating shrinks and compacts the particles, as whey is drained off. The curd particles are pressed together and they fuse to make a uniform body of cheese.
If homogenized milk is used where the fat globules are considerably smaller, the width of the junctions is markedly reduced.
The junctions are visible even to a naked eye. Brick cheese (left figure below) made from curd obtained by knife cutting shows granules relatively similar in size. Such images may be obtained even at a high school chemical laboratory and instructions on how to proceed are given below. They are also characteristic of other 'stirred-curd' cheeses such as Farmer's, Eidam, Gouda, etc.
Also mechanized and automated cheddaring produces both kinds of junction. Different equipment leaves its marks in the junction patterns. Do these findings have any practical importance? Yes. For example, they show that any attempts to alter cheese processing would easily be detected. The findings ensure that the higher price which the consumers pay for Cheddar cheese compared to Farmer's cheese is justified and that they buy a product which really is Cheddar cheese.
Readers who would like to see the junctions in their piece of cheese and who have access to a chemical laboratory, will find that the procedure is relatively simple.
Procedure: Obtain thin (~2 mm) slices of the cheese under study, about 5x5 cm (2"x2") large, and immerse them in an aqueous 2 to 5% glutaraldehyde solution in separate petri dishes overnight. This treatment will fix the cheeses and make them easy to handle. It will also increase the contrast between the junctions and the curd interior. Next day, replace the glutaraldehyde solution with 96% denatured alcohol, about 3 times after 30 minute periods. Then replace ethanol with n-hexane or acetone (twice) to extract fat from the cheese. Using a pair of tweezers, place the cheese slices between two filter papers, place them, with the papers, between two glass or metal plates, place a weight on them, and let the cheese slices dry overnight. All this work must be done in a fume hood. When the cheese slices are dry, they are light and brittle. Sand them carefully with a fine sand paper and see the junctions emerge as sanding progresses. Why is it necessary to sand the slices? Because cutting had smeared the cheese protein on the slices and it is necessary to remove it. Stretched Mozzarella, cheeses with very low fat contents, and processed cheeses do not reveal curd junction patterns. Processed cheese has a relatively short history. First experiments started at the end of the last century but success was achieved only in 1912, when citric acid was introduced as a melting salt. This happened in Switzerland. A few years later, sodium phosphates were added to sodium citrate and have been used since that time.
The initial idea of processing cheese was to increase the shelf life of cheese and, more importantly, to utilize cheeses which may have had various defects. If it was possible with butter by rendering it, some people believed, it should also be possible with cheese. However, when cheese is melted without any additive, fat separates from protein and the result is terrible. The secret of 'processing' cheese is in keeping the fat in the protein matrix. Heating, however, decreases the ability of the cheese proteins to keep the fat globules in the dispersed state, which means that the emulsifying capability of the proteins has been reduced. Melting salts restore it by binding (sequestering) calcium which is present in the caseins. Melting salts with very strong calcium-binding ability (affinity for calcium) lead to the production of hard processed cheeses which contain fat in the form of very small globules. For those readers who like chemistry, the affinity increases in the following order:
Na2HPO4 (disodium phosphate) Na2H2P2O7 (disodium pyrophosphate) Na3HP2O7 (trisodium pyrophosphate) Na4P2O7 (tetrasodium pyrophosphate) Na5P3O10 (pentasodium tripolyphosphate). It has to be emphasized that the melting salts are not emulsifiers but they restore the emulsifying ability of the milk proteins very efficiently. The principles of cheese processing are simple: Various natural cheeses are shredded and then blended with the melting salts and other ingredients such as various kinds of milk solids such as milk powder, whey powder, coprecipitates, cream, butter or butter oil, and sometimes also previously processed cheese. Vegetables and spices may also be added and some processed cheeses may contain 'muscle food ingredients' such as ham, salami, or fish. Other additives such as preservatives, colouring and flavouring agents, binders, and salt and water complete the list of the ingredients. The blend is heated with constant stirring until a smooth mass is formed. In a continuous processed cheese production, the temperature is increased to 140°C for a few seconds to destroy harmful bacteria (such as clostridia) if they happen to be present in some of the ingredients.
From the structural viewpoint, many features characteristic of natural cheeses are destroyed, for example, the curd granule junction patterns and the original fat globule membranes. On the other hand, new features are formed. In most processed cheeses, undissolved melting salt crystals may still be evident. Adding the salts in crystalline form rather than in the form of an aqueous solution to the cheese blend leaves some crystals undissolved. Interactions between the melting salts and calcium in the natural cheeses lead to the formation of insoluble calcium phosphates. Emulsification of fat - that means disintegration of large fat globules into smaller droplets - is also often noticeable in processed cheese. Processed cheese rework
The amount of heat absorbed by processed cheese blends during processing may vary and may even be excessive at times. A blend may receive too much heat during continuous processing if, for example, packaging is delayed for some reason and the flow of the viscous processed cheese blend in the pipes is reduced. The blend eventually thickens and stops moving. Then it is called hot melt. It is removed from the pipes and is frozen for future use. Reworking or reprocessing consists of thawing and shredding the hot melt and adding a small quantity of it to a fresh blend. This is often done on purpose to modify the melting properties of processed cheese in a desired manner. Hot melt is thus a type of process cheese food that is not packaged for sale although it would meet product specifications. If it is re-used, it is called rework. Electron microscopy revealed structural changes in the proteins in rework in the form of small (<1 &micr;m in diameter) dark areas in the micrographs of thin sections. Darkening may be the result of compaction of the cheese proteins or alterations in their chemical structure whereby the heat-modified proteins would react more intensively with heavy metals used during fixation and staining. Tests, in which heavy metals (Os, Pb, U) were omitted from the fixatives and stains during sample preparation for electron microscopy indicated that the proteins in the dark areas were rather compacted than chemically altered. The submicrostructure of the compacted areas was found to be related to the melting salt used to make the processed cheese. Hot melt contained considerably less undissolved melting salt crystals apparently because the crystals had time to dissolve during the entended exposure to heat. Rework dispersed rapidly in the freshly processed cheese blend. This visual observation was confirmed by optical microscopy. The upper image at left shows compact electron-dense structures (purple arrow) which developed in processed cheese made with 2.7% trisodium phosphate, used as the melting salt, due to excessive heating (82°C for 5 hours). The lower image at left shows two kinds of electron-dense structure developed in process cheese made with 20% rework consisting of hot melt. The hot melt was obtained by processing cheese with 2.7% sodium citrate and heating it at 82°C for 5 hours (green arrow). The shredded hot melt was added to a fresh cheese blend and the mixture was processed (using 2.7% trisodium phosphate) and excessively heated. Purple arrows point to structures developed in the fresh cheese blend. What do these findings mean in processed cheese production? They show that the loss of meltability is associated with structural changes in cheese proteins. They make it possible to detect if rework was used in cheese processing; even at 10% rework, there was a high concentration of the dark areas in the micrographs. Finally, these findings point to interesting thermal effects on processed cheese, which may eventually be studied in greater detail and explained.
Scanning electron microscopy of cheese How to prepare cheese samples for SEM
As with any sample to be examined by electron microscopy, the scientist/technician must have knowledge of the composition and structure of the material to be examined. Curd and cheese consist of a milk protein matrix which is interspersed with fat globules and lactic acid bacteria from the starter culture. Other structures such as calcium phosphate crystals or ingredients used in the cheese production may also be present. As curd changes into cheese, the matrix become gradually more compact which means that whey and air pockets are reduced in size. It also means that fixation, dehydration, and defatting is decelerated as the diffusion of the respective agents is hampered.
The original paper by Allan-Wojtas and Kaláb on yogurt may be difficult to obtain. The procedure may be adapted for cheese. It is easy to check as to whether a 24-h postfixation with osmium tetroxide was sufficient: when fractured, the sample should be dark throughout its entire diameter. However, it is not necessary to wash the samples for 48 h as mentioned in the text from the Electron Microscopy section reproduced below:
|
| Author: M. Kaláb |


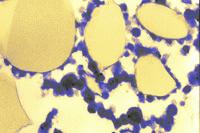 The removal of the whey makes the casein matrix (shown blue to black) more compact and also brings the fat globules (yellow) closer together. The curd shown was made from fresh full-fat milk. The fat globules appear as separate entities in the curd - they do not interact with the casein micelles in the fresh milk and they may be characterized as nonreacting inclusions in the curd. This is true of fat globules unaffected by homogenization.
The removal of the whey makes the casein matrix (shown blue to black) more compact and also brings the fat globules (yellow) closer together. The curd shown was made from fresh full-fat milk. The fat globules appear as separate entities in the curd - they do not interact with the casein micelles in the fresh milk and they may be characterized as nonreacting inclusions in the curd. This is true of fat globules unaffected by homogenization.
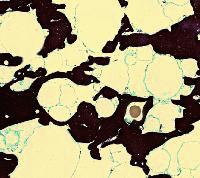
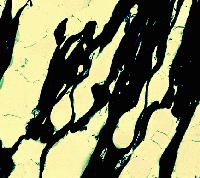 The increase in the density of the curd matrix as a result of whey removal and pressing has been followed in various cheeses by TEM. The micrograph at left shows a thin section of cheddared curd in a cross section. The casein micelle clusters are now more compacted (dark areas) than in the preceding micrographs. The fat globules are in contact with each other, having retained their fat globule membranes. An occasional bacterium (brown) may also be found. Cheddaring is a process, during which slabs of the warm curd are piled up in the cheese vat, subjecting the curd to a slow flow. It aligns the proteins and fat globules into a 'fibrous' structure reminiscent of a baked chicken breast. A section cut parallel with the 'fibres' shows the internal organization of the curd (micrograph at right). Similar kinds of structuring ('stretching') may be found in Italian-style Mozzarella cheese and, in particular, in 'string cheeses'.
The increase in the density of the curd matrix as a result of whey removal and pressing has been followed in various cheeses by TEM. The micrograph at left shows a thin section of cheddared curd in a cross section. The casein micelle clusters are now more compacted (dark areas) than in the preceding micrographs. The fat globules are in contact with each other, having retained their fat globule membranes. An occasional bacterium (brown) may also be found. Cheddaring is a process, during which slabs of the warm curd are piled up in the cheese vat, subjecting the curd to a slow flow. It aligns the proteins and fat globules into a 'fibrous' structure reminiscent of a baked chicken breast. A section cut parallel with the 'fibres' shows the internal organization of the curd (micrograph at right). Similar kinds of structuring ('stretching') may be found in Italian-style Mozzarella cheese and, in particular, in 'string cheeses'.
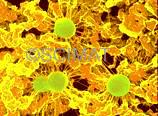 Cottage cheese represents an early stage product in the cheese manufacture. Curdled milk, cut into cubes, is heated and gently stirred. The cubes shrink and expel whey, as has already been explained. The whey is drained off and the curd is cooled so that its grains are prevented from 'matting', i.e., fusing with each other. The microstructure of Cottage cheese, as seen by SEM at left, is still porous - consisting of distinct casein micelle clusters. Three bacteria (greenish) can be seen as if attached by filaments to the protein network. These filaments developed during sample preparation from a thin layer of polysaccharide mucus (bacterial capsules) which surrounded the bacteria in the fresh curd. Highly hydrated polysaccharides are produced by some lactic acid bacteria. They are beneficial in our diet as a source of dietary fibre. In addition, the high viscosity of these polysaccharides modifies the mouthfeel of the food products such as Cottage cheese or yogurt. A high water-holding capacity of the bacterial polysaccharides is another beneficial property. However, the polysaccharides cannot be fixed during preparation of the specimen for electron microscopy and the mucus shrinks during dehydration in ethanol into filaments (at higher mucus concentrations it would shrink into scales). The filaments thus did not exist in the original Cottage cheese and are artifacts.
Cottage cheese represents an early stage product in the cheese manufacture. Curdled milk, cut into cubes, is heated and gently stirred. The cubes shrink and expel whey, as has already been explained. The whey is drained off and the curd is cooled so that its grains are prevented from 'matting', i.e., fusing with each other. The microstructure of Cottage cheese, as seen by SEM at left, is still porous - consisting of distinct casein micelle clusters. Three bacteria (greenish) can be seen as if attached by filaments to the protein network. These filaments developed during sample preparation from a thin layer of polysaccharide mucus (bacterial capsules) which surrounded the bacteria in the fresh curd. Highly hydrated polysaccharides are produced by some lactic acid bacteria. They are beneficial in our diet as a source of dietary fibre. In addition, the high viscosity of these polysaccharides modifies the mouthfeel of the food products such as Cottage cheese or yogurt. A high water-holding capacity of the bacterial polysaccharides is another beneficial property. However, the polysaccharides cannot be fixed during preparation of the specimen for electron microscopy and the mucus shrinks during dehydration in ethanol into filaments (at higher mucus concentrations it would shrink into scales). The filaments thus did not exist in the original Cottage cheese and are artifacts.
 All have some features in common: the milk is first heated to at least 85°C and then is coagulated using an acid such as citric, lactic, acetic, or hydrochloric acid (or an acid precursor such as glucono-δ-lactone) to a final pH value of 5.5 making the curd mildly acidic. The coagulated milk is then cooled and the whey is separated. The microstructure of the casein particles has a characteristic 'core-and-shell' structure (micrograph at left).
All have some features in common: the milk is first heated to at least 85°C and then is coagulated using an acid such as citric, lactic, acetic, or hydrochloric acid (or an acid precursor such as glucono-δ-lactone) to a final pH value of 5.5 making the curd mildly acidic. The coagulated milk is then cooled and the whey is separated. The microstructure of the casein particles has a characteristic 'core-and-shell' structure (micrograph at left).
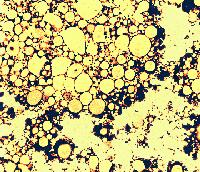
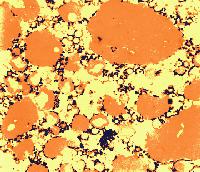
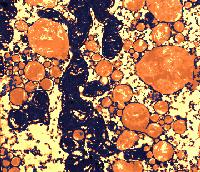
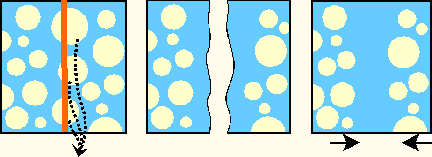 The sites of contact, where adjoining curd particles meet, are called curd granule junctions. Their development is schematically shown in the diagram. The gelled (nonhomogenized) milk which contains fat globules (yellow disks) is cut (red vertical line). The fat globules thus exposed are washed out from the curd (arrows). The surface of the granules heals (middle figure). Pressing of the 2 granules together (last figure) causes the superficial layers depleted of fat to fuse. The junction is shown as an area devoid of fat globules.
The sites of contact, where adjoining curd particles meet, are called curd granule junctions. Their development is schematically shown in the diagram. The gelled (nonhomogenized) milk which contains fat globules (yellow disks) is cut (red vertical line). The fat globules thus exposed are washed out from the curd (arrows). The surface of the granules heals (middle figure). Pressing of the 2 granules together (last figure) causes the superficial layers depleted of fat to fuse. The junction is shown as an area devoid of fat globules.
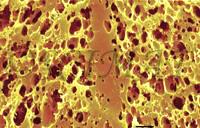 The junctions can easily be seen by scanning electron microscopy (SEM) as compact zones. To obtain the micrograph shown, a small Brick cheese sample (1x1x10 mm) was fixed in a glutaraldehyde solution, dehydrated in ethanol, defatted in n-hexane, returned into absolute ethanol, and rapidly frozen in liquid Freon 12. The frozen sample was transferred into liquid nitrogen, where it was freeze-fractured, returned into absolute ethanol, where it thawed, and then it was critical-point dried from liquid carbon dioxide. Defatting and freeze-fracturing were the essential steps to show the junctions. Removal of fat makes the fracture plane of the sample 'rough' - full of cavities (initially occupied by fat) - in the area showing the interior of the curd granules. The protein walls separating the cavities scatter light in all directions. Light scattering causes this area to appear lighter than the compact structure of the curd granule junctions, which is mostly free of fat globules. The contrast between both structures creates the curd granule junction patterns.
The junctions can easily be seen by scanning electron microscopy (SEM) as compact zones. To obtain the micrograph shown, a small Brick cheese sample (1x1x10 mm) was fixed in a glutaraldehyde solution, dehydrated in ethanol, defatted in n-hexane, returned into absolute ethanol, and rapidly frozen in liquid Freon 12. The frozen sample was transferred into liquid nitrogen, where it was freeze-fractured, returned into absolute ethanol, where it thawed, and then it was critical-point dried from liquid carbon dioxide. Defatting and freeze-fracturing were the essential steps to show the junctions. Removal of fat makes the fracture plane of the sample 'rough' - full of cavities (initially occupied by fat) - in the area showing the interior of the curd granules. The protein walls separating the cavities scatter light in all directions. Light scattering causes this area to appear lighter than the compact structure of the curd granule junctions, which is mostly free of fat globules. The contrast between both structures creates the curd granule junction patterns.

 Cheddar cheese manufacture involves considerably more work. The fused curd granules (curd slabs) are piled one over the other in the cheese vat in traditional Cheddar making. Heat and the presence of fat in the curd make it to slowly flow down. This cheddaring process elongates the granules. The slabs are gradually replaced so that all are exposed to maximum flow and then the slabs are milled into finger-like pieces. These are salted and pressed together. Milling produces new cuts and thus new junctions (right figure). They are called milled curd junctions and are noticeably thicker than the curd granule junctions.
Cheddar cheese manufacture involves considerably more work. The fused curd granules (curd slabs) are piled one over the other in the cheese vat in traditional Cheddar making. Heat and the presence of fat in the curd make it to slowly flow down. This cheddaring process elongates the granules. The slabs are gradually replaced so that all are exposed to maximum flow and then the slabs are milled into finger-like pieces. These are salted and pressed together. Milling produces new cuts and thus new junctions (right figure). They are called milled curd junctions and are noticeably thicker than the curd granule junctions.
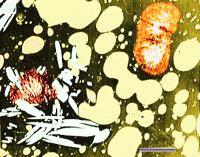
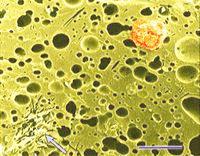
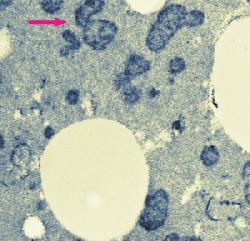
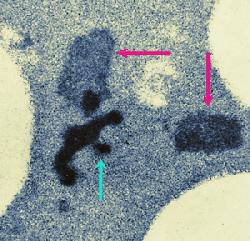 Overheating affects the microstructure of processed cheese.
Overheating affects the microstructure of processed cheese.