Food Structure Journal 1982-1993:
Foods under the microscope: 2. Fats and oils
Fats and oils are indispensable parts of human nutrition. They are the main energy sources for the body. There is more energy contained in a g of fat or oil (9 calories/gram) than in proteins (4 cal/g) and in glycides (4 cal/g). It is consumed in the form of fats of animal origin as pork fat, lard, chicken fat, fish oil and as oils of plant origin such as olives, avocado, seeds such as poppy seeds, linseed, sunflower seeds, nuts etc. Large amounts as fats and oils are consumed in the form of margarine, butter, vegetable oils and also as milkfat in the form of dairy products. Fats and oils are also used as spreads and dressings and for baking and frying. Fats and oils are substances consisting of glycerol associated with fatty acids. The difference between fats and oils is mainly in the composition of the fatty acids. Unsaturated fatty acids found in vegetable oils and fish oil produce liquid oils whereas saturated fatty acids are part of fats.
|
The subject of this presentation is the microstructure of foods with emphasis on scientific papers published in the Food Microstructure - Food Structure 1982-93 journal. Part 1 reviews the microstructure of Milk and dairy products and the discontinued journal. Part 2 is about Fats and oils. References to other sources are also listed in related topics. Food scientists who study other foods such as cereals, meat, legumes, fruits and vegetables etc. are invited to contribute their fields of interest in a similar manner. The Editor
|
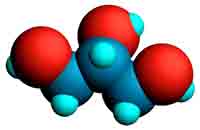
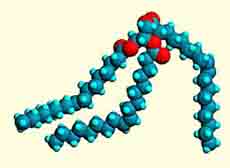
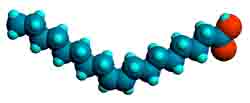
Fats and oils are mixtures of various triglycerides. They consist of an alcohol, glycerol (Fig. 1, left image) with fatty acids such as oleic acid (Fig. 2, right image) attached in the form of ester bonds (Fig. 1, centre). The molecular model shown (centre) is called triolein because it consists of 3 oleic acid molecules bound to glycerol. Oleic acid is a mono-unsaturated fatty acid because it contains one double bond in CIS configuration which means that the neighbouring carbon atoms are positioned on the same side of the chain in contrast to the TRANS configuration.
|
|
|
|
Fig. 1. Molecular models of glycerol (left), triolein (middle), and oleic acid
(right). Carbon atoms: gray; hydrogen: cyan; and oxygen: red.
|
Optical microscopy and electron microscopy are used to visualize fats and oils in foods and other biological materials. Whereas stains such as Oil Red O, Sudan Black, Nile Blue etc. used in optical microscopy impart various colours to lipids (fats and oils), fixation and staining for transmission electron microscopy (TEM) of thin sections is based on stabilizing the lipids and attaching electron-dense substances such as heavy metals (e.g., osmium) to them. However, since osmium tetroxide, as one of the best fixatives for fats and oils, separates from unsaturated fatty acids in aqueous media, imidazole is used to attach it firmly as these equations show.
|
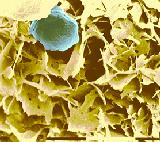
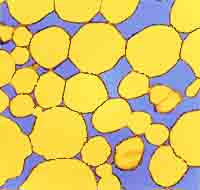
Fig. 2. Margarine consists of
a crystalline network (yellow), water droplets (their shell is shown in blue) and the liquid oil portion (removed in order to make cryo-SEM possible).
|
For scanning electron microscopy (SEM) of the crystalline part of fats and oils, there is a need to remove the liquid (oil) phase of the fat under study. The method was described by Heertje et al. (FM 6(1) 1-8, 1987). It consists of replacing the oil phase (de-oiling) with a mixture of 2-butanol and methanol (90/10; v/v) in a sintered bronze holder, freezing, freeze-drying, fracturing, coating with gold, and transferring into the microscope. The authors described a special type of a sample preparation holder. De-oiling was done between 5 and 15°C for 15 to 45 h depending on the water content in the fatty product, dimensions of the fat crystals and de-oiling temperature. The de-oiled sample was transferred into 100% methanol, kept in it at the de-oiling temperature for 24 h, frozen in liquid nitrogen, and freeze-dried (removing methanol) at 173 K. This temperature is critical: it allows the sublimation of solid methanol at a reasonable rate, yet the crystalline matrix of the sample is not affected. Fig. 2 at left shows a typical margarine structure of a crystalline network and water droplet shells, which also consist of fat crystals.
Freeze-fracturing (sometimes followed by freeze-etching if an aqueous phase is also present and there is a need to emphasize the lipid component) and subsequent replication of the fractured specimens with platinum and carbon is one of the best procedures to study the microstructure of crystalline lipids. The methods were described in detail by W. Buchheim (FM 1(2) 189-208, 1982) with warnings about potential artifacts which mostly arise from ice crystal formation in the aqueous phase present and from difficulties associated with cleaning the replicas.
W. Buchheim published his numerous findings in Food Microstructure, Food Structure (see the References) and also elsewhere. In their paper Milk and dairy type emulsions, W. Buchheim and P. Dejmek also studied fat components of cream liqueurs and coffee whiteners and have shown their micrographs.

|
| Fig. 3. The air-water interface of a bubble in a foam produced by mixing egg and shortening. Numerous fat crystals are adsorbed at the interface. (Pt/C replica, TEM; bar: 1 µm)). (Brooker, FS 12(3), 285-296, 1993).
|
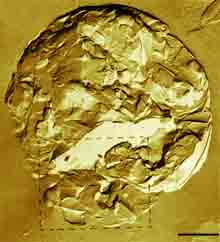
|
| Fig. 4. A freeze-fractured fat globule in cream. (Bar: 1 µm) (deMan, FM 1(2), 209-222, 1982 – From W. Buchheim & D. Precht, Milchwissenschaft 34, 657-662, 1979.
|

|
| Fig. 5. Crystal structure of fat in 10 days old butter. (Bar: 0.1 µm) (deMan, FM 1(2), 209-222, 1982) – From D. Precht & W. Buchheim, Milchwissenschaft 35, 393-398, 1980.
|
Figs. 3, 4 and 5 above are examples of micrographs obtained by freeze-fracturing of fat crystals in fats and oils and of the internal crystalline microstructure of fat globules in various foods.
I. Heertje reviewed microstructural studies in fat research (FM 12(1) 77-94, 1993):
Shortenings are the simplest culinary fats because they are composed only of liquid oil and solid fat crystals. Extracting the oil reveals a 3-dimensional network of saturated fat crystals. Margarine and butter are more complex as they contain, in addition to oils and fats, also 20% of water. The water is present in the form of minute droplets, several micrometers in diameter, each covered with a shell of fat crystals.
Margarine is also composed of a continuous network structure of fat crystals and their aggregates but it differs from shortenings by the presence of water droplets (Fig. 2). Water droplets several micrometers in diameter are formed during intensive mixing of fat and water phases during processing. Crystals orientate at the water droplet surface and stabilize the water droplet. The continuous fat matrix shows an interconnected network structure composed of plate-like crystal aggregates.
Butter has a discontinuous structure also consisting of fat globules which interact very little with the other components or only to a limited extent. Many consumers prefer the taste and the mouthfeel of butter over margarine. This is because the differences in structure between margarine and butter are reflected in their functional properties. For the manufacturers of margarine, understanding of the relationship between structure and function offers possibilities of adjusting the structure in such a manner that desired functional properties can be obtained. I. Heertje concludes that this is, in fact, one of the main objectives for doing microstructural research.
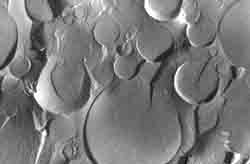
|
| Fig. 6. Mayonnaise. A thin section examined by TEM shows oil globules (yellow).
|
Mayonnaise is a stable oil-in-water emulsion consisting of vegetable oil, vinegar, and an emulsifier such as egg yolk. In commercial mayonnaise, vinegar may be replaced with lemon juice and egg yolk-free spread resembling mayonnaise is available to consumers who wish to avoid egg yolk for various reasons. It is actually lecithin in the egg yolk which has the emulsifying property.
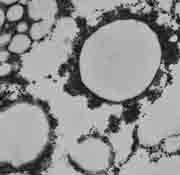
|
| Fig. 7. Mayonnaise - freeze-fractured and replicated with Pt and C.
|
To study the microstructure of mayonnaise by TEM of thin sections, a small amount is encapsulated in agar gel tubes (1.0-1.5 mm in diameter, prepared from an agar sol at 40°C or better, in a calcium alginate gel at low temperature (Encapsulation of viscous high-fat foods in calcium alginate gel tubes at ambient temperature. I. A. Veliky, M. Kaláb. FS 9(2) 151-154, 1990.). Imidazole must be part of the osmium tetroxide fixative, otherwise a part of the oil globules would be lost during the subsequent preparatory steps. Fig. 6 shows that all oil globules are covered with membranes which formed during emulsification. A similar phenomenon also occurs when milk is homogenized, i.e., milkfat globules are broken into small ones. Milk proteins immediately form new fat globule membranes on the freshly made surfaces.
Freeze-fracturing of a mayonnaise specimen at -100°C and replication with platinum and carbon (Pt/C) produced a replica that was lifted from the specimen, cleaned, and examined by TEM (Fig. 7).
Tung and Jones (Studies of Food Microstructure, pp. 231-238, also SEM/1981/III:523-530) studied mayonnaise by optical microscopy and by SEM and statistically evaluated the mean diameters of the oil globules in two different brands (2.21 and 2.24 µm) with very high standard deviations (2.18 and 2.60 µm, resp.). The discussion with the reviewers would interest any food microscopist visiting this subject.
|
| Fig. 8. Fat globules in cream cheese are covered with milk proteins. (TEM of thin sections).
|
Micrographs of fat in milk products show several interesting phenomena. The aggregation of milk proteins on disrupted milkfat globules visible in detail in Fig. 8 at left is illustrated on page 22 in Fig. 11 of the article on the Beauty of Milk at High Magnification. However, there are marked differences in the microstructures and sensory attributes of commercial cream cheeses made using different technological processes. Traditional cream cheese made from cream ripened with a lactic bacterial culture was composed of fat globule clusters with their membranes on the surfaces mostly preserved.
In the 80ies of the 20th century, the manufacture of cream cheese was simplified by mixing cream or anhydrous milkfat with other milk solids including acid-coagulated curd and the mixture was pasteurized and homogenized. Large fat particles prevailed over small fat globules (Electron microscopy and sensory evaluation of commercial cream cheese. Studies of Food Microstructure, 53-162, also SEM/1981/III:473-482, 514.
Development of microstructure in a cream cheese based on Queso Blanco cheese. FM 4(1) 89-98, 1985). Electron microscopy of thin sections also distinguished between low-fat Neufchâtel cheese and imitation cream cheese spreads made using vegetable oil.

|
| Fig. 9. Saturated fat crystallized in a milkfat globule in cream cheese kept in a refrigerator. (TEM of thin sections).
|
Thermal history of fats and oils may be seen from micrographs. Unless these foods are kept in a refrigerator at a low temperature of 4-6°C, no crystals develop in fat of animal origin. Thin sections show a homogeneous content of the fat globules. When such fat cools, saturated fat crystalizes. Osmium tetroxide in the presence of imidazole reacts with the unsaturated portion but not with saturated fatty acid crystals. Heavy metal salts such as uranyl acetate and lead citrate used as stains also do not react with the crystals. Consequently, such crystals appear as light areas in the micrographs (Fig. 9). They are shown in greater detail on page 15, Fig. 7, in The Beauty of Milk at High Magnification.
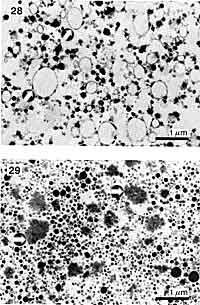
|
| Fig. 10. A comparison of a super premium ice cream mix (top) consisting of milkfat globules (arrows) and casein micelles (dark dots) and a fat-free ice cream mix (bottom) containing milk and egg white microparticulated proteins (arrows). (TEM of thin sections).
|
Fat substitutes or fat replacers are of various kinds. They mimic some roles of fat in a food. A description of their composition and properties lists Caprenin, Salatrim, and Olestra as fat-based substitutes, Simplesse as the major protein-based fat substitute, and Avicel, carrageenan, dextrins, and inulin as carbohydrate-based fat substitutes.
Simplesse is interesting from the microstructural viewpoint for several reasons. It had been developed by N. S. Singer (Protein microparticulation: The principle and process. N. S. Singers and J. M. Dunn. J. Am. Coll. Nutr. 9, 388-397, 1990) from coagulated milk and egg white proteins in the form of globular particles about 1 µm in diameter. Particles of these dimensions are perceived by the human tongue as "fat" irrespective of their composition. This means that even brick, rock, or steel particles 1 µm in diameter would feel like fat in the mouth. – Milk proteins are considered as natural food to most people while egg white proteins have eventually been omitted from Simplesse. However, even the milk and egg proteins have to be listed as ingredients if they have been used. –

|
| Fig. 11. Hard-boiled egg yolk has a granular structure which may be observed by light microscopy.
|
Microparticulated proteins may be used only in foods which are not fried or baked, that means salad dressings, ice cream, cheese etc. Fig. 10 shows micrographs of a "super premium" ice cream mix consisting of fat globules (top) and a fat-free ice cream mix containing microparticulated milk and egg white proteins instead of milkfat. Ice cream samples made from both mixes were evaluated as of equal quality by a skilled team of ice cream tasters.
Fat globules present in other foods. Using microscopy, M. M. Perry and A.B. Gilbert described tightly packed spheres in hen's egg yolk. The yolk contains all the fat present in the egg. Boiling coagulates both the egg white and the egg yolk. Compared to the smooth structure of the egg white (see the bottom of I. Heertje's study), the egg yolk appears to be granular. Light microscopy reveals the structure of the granules (Fig. 11).
Ice cream microstructure is explained in the Food Structure 1982-1993 Part 1.
Compilation of this presentation on the microstructure of fats and oils is further in progress.
References to papers published in Food Microstructure (FM) and Food Structure (FS):
(Readers may find the complete Tables of Contents at several places. Librarians in academic and industrial research establishments may locate the nearest library holding the journal through their regular channels including OVID Technologies).
• Some effects of lipids on the structure of foods. K. Larsson (Review paper), FM 1(1) 55-62, 1982.
• Aspects of sample preparation for freeze-fracture/freeze-etch studies of proteins and lipids in food systems. W. Buchheim (Review paper). FM 1(2) 189-208, 1982.
• Microscopy in the study of fats and emulsions. J. M. deMan (Review paper). FM 1(2) 209-222, 1982.
• Electron microscopic localization of solvent-extractable fat in agglomerated spray-dried whole milk powder particles. W. Buchheim. FM 1(2) 233-238, 1982
•
Development of microstructure in a cream cheese based on Queso blanco cheese. M. Kaláb, H. W. Modler. FM 4(1) 89-98, 1985.
• Relation between microstructure, destabilization phenomena and rheological properties of whippable emulsions. W. Buchheim, N. M. Barfod, N. Krog. FM 4(2) 221-232, 1985.
• Combining microscopy and physical techniques in the study of cocoa butter polymorphs and vegetable fat blends. J. D. Hicklin, G. G. Jewell, J. F. Heathcock. FM 4(2) 241-248 1985.
• Crystal morphology of cocoa butter. D. M. Manning, P. S. Dimick (Review paper). FM 4(2) 249-266, 1985.
• Observations on the air-serum interface of milk foams. B. E. Brooker. FM 4(2) 289-296, 1985.
• Microscopical observations on the structure of bacon. C. A. Voyle, P. D. Jolley, G. W. Offer (Review paper). FM 5(1) 63-70, 1986.
• Ultrastructural aspects and physico-chemical properties of ultrahigh-temperature (UHT)-treated coffee cream. W. Buchheim, G. Falk, A. Hinz. FM 5(1) 181-192, 1986.
• The development of structure in whipped cream. B. E. Brooker, M. Anderson, A. T. Andrews. FM 5(2) 277-286 1986.
• Product morphology of fatty products. I. Heertje, M. Leunis, W. J. M. Van Zeyl, E. Berends. FM 6(1) 1-8, 1987.
• Physical and molecular properties of lipid polymorphs - a review. K. Sato (Review paper). FM 6(2) 151-160, 1987.
• The effects of polysorbate 80 on the fat emulsion in ice cream mix: Evidence from transmission electron microscopy studies. H. D. Goff, M. Liboff, W. K. Jordan, J. E. Kinsella. FM 6(2) 193-198, 1987.
• Microstructure of shortenings, margarine and butter - a review. A. C. Juriaanse, I. Heertje (Review paper). FM (7(2) 181-188, 1988.
•
The effect of processing on some microstructural characteristics of fat spreads. I. Heertje, J. van Eendenburg, J. M. Cornelissen, A. C. Juriaanse. FM 7(2) 189-194, 1988.
•
Observation of seeding effects on fat bloom of dark chocolate. I. Hachiya, T. Koyano, K. Sato. FM 8(2) 257-262, 1989.
•
Polymorphism of triglycerides - a crystallographic review. L. Hernqvist (Tutorial paper). FS 9(1) 39-44, 1990.
•
Thermographic behavior of coconut oil during wheat dough mixing: Evidence for a solid-liquid phase separation according to mixing temperature. C. Le Roux, D. Marion, H. Bizot, D. J. Gallant. FS 9(2) 123-138, 1990.
•
The adsorption of crystalline fat to the air-water interface of whipped cream. B. E. Brooker. FS 9(3) 223-230, 1990.
•
Fat polymorphism and crystal seeding effects on fat bloom stability of dark chocolate. T. Koyano, I. Hachiya, K. Sato (Review paper). FS 9(3) 231-240, 1990.
•
Crystal morphology of shortenings and margarines. P. Chawla, J. M. deMan, A. K. Smith (Tutorial paper). FS 9(4) 329-336, 1990.
•
Emulsifiers as additives in fats: Effect of polymorphic transformations and crystal properties of fatty acids and triglycerides. J. Aronhime, S. Sarig, N. Garti (Tutorial paper). FS 9(4) 337-352, 1990.
•
Fat-holding in hamburgers as influenced by the different constituents of beef adipose tissue. A. Olsson, E. Tornberg. FS 10(4) 333-344, 1991.
•
The contribution of milk serum proteins to the development of whipped cream structure. E. C. Needs, A. Huitson. FS 10(4) 353-360, 1991.
•
Image analysis to determine intramuscular fat in muscle. T. Ishii, R. G. Cassens, K. K. Scheller, S. C. Arp, D. M. Schaefer. FS 11(1) 55-60, 1992.
•
Computer modeling: The adjunct micro technique for lipids. J. W. Hagemann, J. A. Rothfus (Review paper). FS 11(2) 85-100, 1992.
•
Structure of margarines made with low erucic acid rapeseed oil. J. Hojerová, S. Schmidt, J. Krempaský. FS 11(2) 147-154, 1992.
•
Interfacial viscoelasticity in emulsions and foams. E. H. Lucassen-Reynders (Review paper). FS 12(1) 1-12, 1993.
•
Microstructural studies in fat research. I. Heertje (Tutorial paper). FS 12(1) 77-94, 1993.
•
The stabilisation of air in foods containing fat - a review. B. E. Brooker (Turorial paper). FS 12(1) 115-122, 1993.
•
Microstructure of whey protein/anhydrous milkfat emulsions. M. Rosenberg, S. L. Lee. FS 12(2) 267-274, 1993.
•
The stabilisation of air in cake batters - the role of fat. B. E. Brooker, FS 12(3) 285-296, 1993.
•
Electron microscopy and sensory evaluation of commercial cream cheese. M. Kaláb, A. G. Sargant, D. A. Froehlich. Studies of Food Microstructure pp. 153-162, also SEM/1981/III:473-482, 514.
•
Microstructure of mayonnaise and salad dressing. M. A. Tung, L. J. Jones, Studies of Food Microstructure, pp. 231-238, also SEM/1981/III:523-530.
|












