|
Mounting
bacteria on SEM stubs for long-term preservation
M. Kaláb and A. F. Yang
Double-sided sticky tape has been known to be a useful aid in mounting minute particles such as microorganisms captured on bacterial filters. Following fixation, dehydration, and critical-point drying, the filters (13 mm in diameter) are mounted on double-sided sticky tape attached to aluminum SEM stubs. A conductive silver cement is then used to provide a path from the filter to the stub.
Occasionally a need arises to examine the same specimens again several weeks later. For this reason, it is believed, the specimens should be stored in a dessicator. However, on most occasions, the specimens appear to be useless: bacteria or fungal spores are frequently found glued together. Their surroundings appear dark and the characteristic pores that are seen in fresh polycarbonate Nuclepore or Millipore membrane filters (Figure 1, bar: 10 µm) are severely reduced (Figure 2, bar: 10 µm) or completely missing in the afflicted areas.

 Close examination of what happens when a bacterial filter is positioned on the double-sided sticky tape suggests that the sticky substance first adheres to the back of the filter and keeps it flat on the stub. With time, it is assumed, the sticky substance fills the
pores and gradually oozes out, thus affecting the bacteria or other particles attached to the filter. This would explain the vanishing of the empty pores from
the filter and the clumping of bacteria demonstrated using bifidobacteria (Figure 3, bar: 5 µm).
Close examination of what happens when a bacterial filter is positioned on the double-sided sticky tape suggests that the sticky substance first adheres to the back of the filter and keeps it flat on the stub. With time, it is assumed, the sticky substance fills the
pores and gradually oozes out, thus affecting the bacteria or other particles attached to the filter. This would explain the vanishing of the empty pores from
the filter and the clumping of bacteria demonstrated using bifidobacteria (Figure 3, bar: 5 µm).
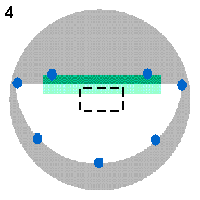
 To check this assumption, a poly- carbonate filter with 0.4 µm pores, 13 mm in diameter, containing a thin layer of Escherichia coli bacteria, was cut in half and mounted on an aluminum SEM stub as shown in Figure 4: A small rectangle (shown in green colour) of a double-sided sticky tape was attached to the stub (gray) and the filter (white) was laid with the cut side over it (light green colour indicates where the filter overlapped over the sticky tape). Several dabs of a conductive silver paint (blue) were applied to attach the filter to the bare stub. Then the filter was sputter-coated with 20 nm of gold and examined in a scanning electron microscope operated at 7.5 kV accelerating voltage.
To check this assumption, a poly- carbonate filter with 0.4 µm pores, 13 mm in diameter, containing a thin layer of Escherichia coli bacteria, was cut in half and mounted on an aluminum SEM stub as shown in Figure 4: A small rectangle (shown in green colour) of a double-sided sticky tape was attached to the stub (gray) and the filter (white) was laid with the cut side over it (light green colour indicates where the filter overlapped over the sticky tape). Several dabs of a conductive silver paint (blue) were applied to attach the filter to the bare stub. Then the filter was sputter-coated with 20 nm of gold and examined in a scanning electron microscope operated at 7.5 kV accelerating voltage.
 The stub was stored at 22°C in a dessicator and examined 3 weeks later
again. A micrograph (Figure 5) shows part of an area where the filter was partly
attached to the sticky tape and partly was lying loose on the stub (as shown by a dashed rectangle in Figure 4).
The micrograph (Figure 5) has been turned so that the border between both areas is running as a diagonal. The bacteria on the loose filter (lower right corner) are scattered individually over the filter and their outlines are sharp in a manner seen at all sites accessible from the "Starting point to food structure studies". The bacteria on the filter over the sticky tape (upper left corner) appear to be surrounded by some "viscous" substance which also glues some of them together and to the filter. The filter pores are less numerous and the remaining ones are smaller than in the other part of the micrograph. Bar: 2 µm.
The stub was stored at 22°C in a dessicator and examined 3 weeks later
again. A micrograph (Figure 5) shows part of an area where the filter was partly
attached to the sticky tape and partly was lying loose on the stub (as shown by a dashed rectangle in Figure 4).
The micrograph (Figure 5) has been turned so that the border between both areas is running as a diagonal. The bacteria on the loose filter (lower right corner) are scattered individually over the filter and their outlines are sharp in a manner seen at all sites accessible from the "Starting point to food structure studies". The bacteria on the filter over the sticky tape (upper left corner) appear to be surrounded by some "viscous" substance which also glues some of them together and to the filter. The filter pores are less numerous and the remaining ones are smaller than in the other part of the micrograph. Bar: 2 µm.
The authors hope that this finding could be helpful to colleagues using a double-sided sticky tape, as it could prevent frustration from "the spoilage of bacteria" mounted on SEM stubs that are stored and used at a later date.
Links to SEM of bacteria:
• Improved preservation of bacterial exopolymers for scanning electron microscopy
•
SEM and ESEM in the studies of biofilm formation
• Conventional
scanning electron microscopy of bacteria
• Combination of microscopic techniques reveals a
comprehensive visual impression of biofilm structure and composition
• Bacteria world
• Assessment of bacterial attachment and biofilm formation using SEM
• Evaluation of Electron Microscopic Sample Preparation
methods and imaging techniques for characterization
of cell-mineral aggregates
For comments, questions, and/or amendments please contact the authors.
Updated: September 23, 2013.
©SCIMAT 2013
|

 Close examination of what happens when a bacterial filter is positioned on the double-sided sticky tape suggests that the sticky substance first adheres to the back of the filter and keeps it flat on the stub. With time, it is assumed, the sticky substance fills the
pores and gradually oozes out, thus affecting the bacteria or other particles attached to the filter. This would explain the vanishing of the empty pores from
the filter and the clumping of bacteria demonstrated using bifidobacteria (Figure 3, bar: 5 µm).
Close examination of what happens when a bacterial filter is positioned on the double-sided sticky tape suggests that the sticky substance first adheres to the back of the filter and keeps it flat on the stub. With time, it is assumed, the sticky substance fills the
pores and gradually oozes out, thus affecting the bacteria or other particles attached to the filter. This would explain the vanishing of the empty pores from
the filter and the clumping of bacteria demonstrated using bifidobacteria (Figure 3, bar: 5 µm).

 To check this assumption, a poly- carbonate filter with 0.4 µm pores, 13 mm in diameter, containing a thin layer of Escherichia coli bacteria, was cut in half and mounted on an aluminum SEM stub as shown in Figure 4: A small rectangle (shown in green colour) of a double-sided sticky tape was attached to the stub (gray) and the filter (white) was laid with the cut side over it (light green colour indicates where the filter overlapped over the sticky tape). Several dabs of a conductive silver paint (blue) were applied to attach the filter to the bare stub. Then the filter was sputter-coated with 20 nm of gold and examined in a scanning electron microscope operated at 7.5 kV accelerating voltage.
To check this assumption, a poly- carbonate filter with 0.4 µm pores, 13 mm in diameter, containing a thin layer of Escherichia coli bacteria, was cut in half and mounted on an aluminum SEM stub as shown in Figure 4: A small rectangle (shown in green colour) of a double-sided sticky tape was attached to the stub (gray) and the filter (white) was laid with the cut side over it (light green colour indicates where the filter overlapped over the sticky tape). Several dabs of a conductive silver paint (blue) were applied to attach the filter to the bare stub. Then the filter was sputter-coated with 20 nm of gold and examined in a scanning electron microscope operated at 7.5 kV accelerating voltage.
 The stub was stored at 22°C in a dessicator and examined 3 weeks later
again. A micrograph (Figure 5) shows part of an area where the filter was partly
attached to the sticky tape and partly was lying loose on the stub (as shown by a dashed rectangle in Figure 4).
The micrograph (Figure 5) has been turned so that the border between both areas is running as a diagonal. The bacteria on the loose filter (lower right corner) are scattered individually over the filter and their outlines are sharp in a manner seen at all sites accessible from the
The stub was stored at 22°C in a dessicator and examined 3 weeks later
again. A micrograph (Figure 5) shows part of an area where the filter was partly
attached to the sticky tape and partly was lying loose on the stub (as shown by a dashed rectangle in Figure 4).
The micrograph (Figure 5) has been turned so that the border between both areas is running as a diagonal. The bacteria on the loose filter (lower right corner) are scattered individually over the filter and their outlines are sharp in a manner seen at all sites accessible from the